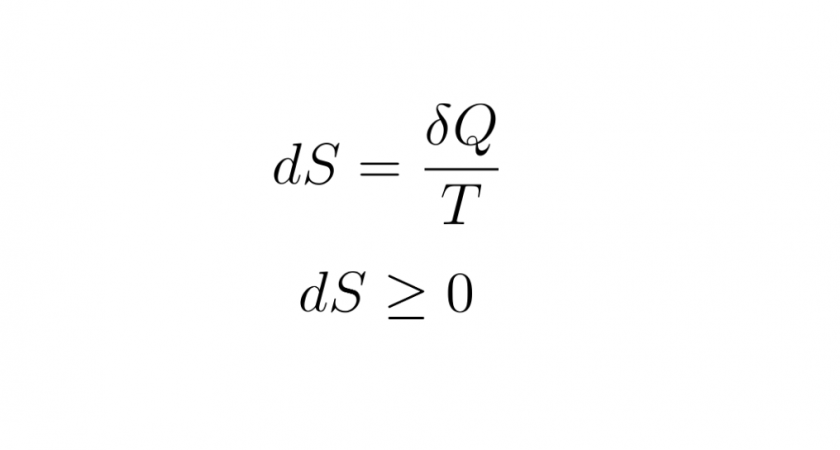Entropy is the unavailability of a system’s thermal energy for conversion into mechanical work.
"Entropy always increases with time." - Sadi Carnot
The Second Law of Thermodynamics states that entropy always increases with time.
The equation for the change in entropy, 𝛥𝑆, is:
𝛥𝑆 = 𝑄𝑇
Q is the heat that transfers energy during a process. T is the absolute temperature at which the process takes place.